Background
Voxelotor is a haemoglobin oxygen-affinity modulator that leads to inhibited red blood cell sickling, has demonstrated reduced markers of haemolysis and anaemia improvement, and is one of a small number of novel disease-modifiers approved in the US for sickle cell disease (SCD) treatment. It has marketing authorisation in the UK and is currently undergoing reimbursement appraisal. The modelled impact of voxelotor on health-related quality of life (HRQoL)remains a key issue in current trial evidence. Therefore, the need for real-world data and understanding of patient-reported outcomes and experiences following treatment remains critical.
Aims
This work sought to characterise the side effects, HRQoL, and wearable-captured sleep and activity metrics of SCD patients in the UK, before and after treatment initiation.
Methods
Feedback from 19 SCD patients who had received voxelotor was captured through an online survey (Aug 2022-Mar 2023). 15 of these patients were also consented and enrolled within an artificial intelligence (AI) platform of over 600 SCD patients that combined genomic, medical record, real-time wearable biometric, and patient-reported outcomes data into a single platform. This included a mobile phone app for day-to-day QoL questionnaire (EQ-5D-5L) entry and an FDA-cleared smartwatch to capture sleep quality and physical activity.
Convenience sampling selected data for a 17-patient (89%) subcohort with available pre- and post-voxelotor initiation EQ-5D-5L scores, either through the platform or follow-up survey snapshots. For each patient, this was analysed up to the date on which they completed the survey. EQ-5D-5L was also analysed in comparison to platform patients who had hydroxyurea (HU) in the past (n=19) or were receiving HU at the time of analysis (n=60), in addition to those with no recorded HU use (n=116). Additional analysis compared pre- and post-initiation activity levels (n=6), 4 of whom also had sleep quality data.
Results
The 19 surveyed patients had received voxelotor for a mean ± SD (range) of 194 ± 415 (17-1,826) days. 11 (58%) had also received HU and 1 (5%) received crizanlizumab. The mean age was 33 ± 13 (14-52) years, 12 (63%) were male, and 16 (84%) had HbSS. Side effects were reported in 8 (42%) patients, mostly diarrhea (6, 32%), stomach pains (2, 11%), and nausea/vomiting (2, 11%).
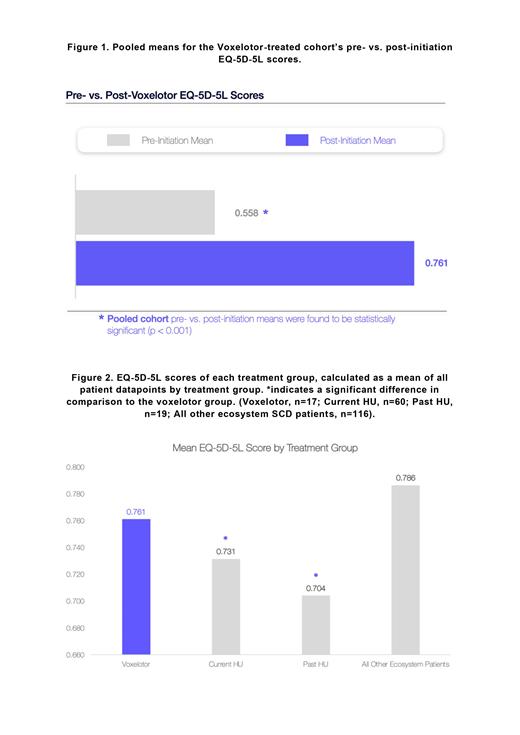
Comparison of the 17-patient pre- to post-voxelotor cohort showed a statistically significant (p<0.001) increase in pooled EQ-5D-5L scores, from 0.558 ± 0.314 to 0.761 ± 0.262 (Figure 1). Post-initiation EQ-5D-5L for these 17 patients was significantly higher than for those receiving HU at 0.731 ± 0.232 (p=0.023) or who had previously received HU at 0.704 ± 0.256 (p=0.040) (Figure 2).
Analysis of pre- and post-initiation wearables data saw increased deep sleep in 50% (2/4) and decreased nightly wakeups in 50% (2/4) of patients. Pooled pre- and post-initiation data showed a significant difference in deep sleep (43% vs. 47%, p=0.011). The mean number of steps and distance increased for 50% (3/6) of patients. While soft activity (Metabolic Equivalent of Task (METs): <3, e.g., sleeping, sitting quietly, slow walk) decreased for 67% (4/6) of patients, moderate activity (MET: 3-6, e.g., fast walk, cleaning, gardening) increased in 67% (4/6) and intense activity (MET: >6, e.g., running, hiking) increased in 60% (3/5).
Conclusions
Our data present a different lens to the voxelotor treatment experience of patients in the UK, with a side effect profile similar to that of other published studies. We demonstrated that EQ-5D-5L increased by 0.203 pre- to post-initiation, and was 0.030 and 0.057 higher than those receiving HU and those who had previously received HU, respectively, in the voxelotor-treated group. These are in line with the ranges of minimally important differences (0.030-0.098) reported for the EQ-5D-5L in other chronic diseases.
As such and despite the limitations of small sample size and variability in pre- to post-initiation changes, a statistically significant improvement in HRQoL was observed after initiating voxelotor, as well as in comparison to HU treatment pathways. Similarly, pre- and post-initiation wearables data demonstrated early positive trends in voxelotor's impacts on sleep and activity using an FDA-cleared device, highlighting a need for further research into these metrics and the impact of SCD-modifying treatment.
Disclosures
Summers:Pfizer Ltd: Other: Employed by Sanius Health, who have a financial work contract with the company. Agrippa:Pfizer Ltd: Consultancy. Shastri:Pfizer Ltd: Current Employment. Sharif:Pfizer Ltd: Current Employment. Anie:Pfizer Ltd: Honoraria. Telfer:Novartis: Other: Advisory board; Clinical trial activity; ; Pfizer: Other: Advisory board; Clinical trial activity; Data monitoring committee; ; Apopharma: Other: Clinical trial activity, Speakers Bureau; Celgene: Other: Clinical trial activity; Napp Pharmaceuticals: Other: Clinical trial activity; Kyowa Kirin Limited: Other: Investigator-led funding; Terumo: Speakers Bureau; bluebird bio: Other: Advisory board; Investigator-led funding;. Lugthart:GBT: Other: Conference attendance.